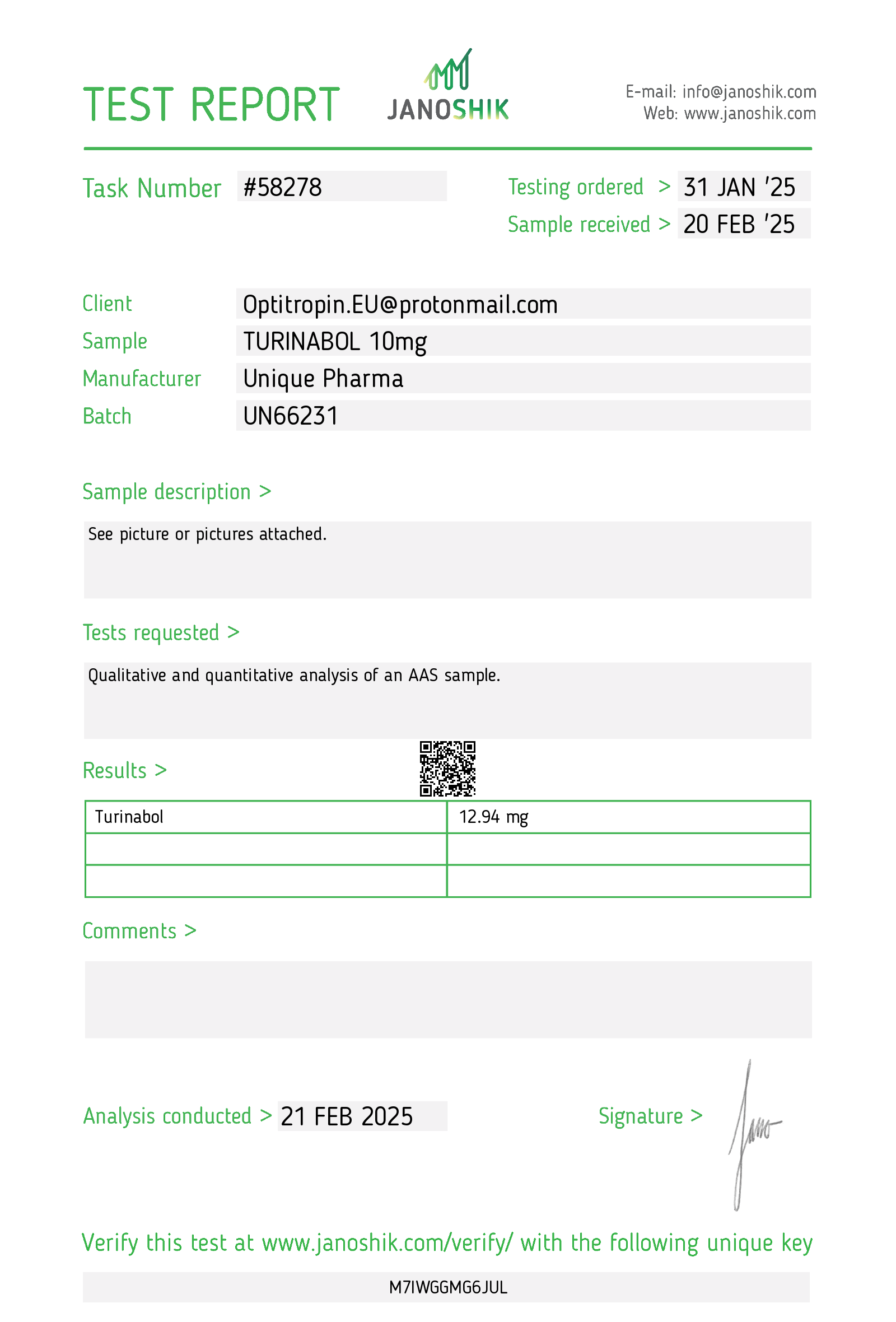
Chemical Profile
- Generic name: Chlorodehydromethyltestosterone (commonly known as Turinabol or Tbol)
- Chemical class: 17α-alkylated anabolic-androgenic steroid
- Molecular formula: C20H27ClO2
- Molecular weight: approximately 334.88 g·mol−1
- Physical form: oral solid dosage (tablets)
- Terminal elimination half-life: approximately 16 hours
Clinical Pharmacology
Turinabol is a modified form of Dianabol with a chlorine atom at the 4-position, which prevents aromatization. This results in lean muscle gains without water retention or estrogenic side effects. The compound has a favorable anabolic to androgenic ratio, making it suitable for both male and female athletes.
Applications
- Lean muscle mass development
- Strength gains without water retention
- Athletic performance enhancement
- Suitable for cutting and lean bulking phases
Dosage Guidelines
Typical dosing ranges from 20-50mg daily for men and 5-10mg daily for women. Due to its 17α-alkylated structure, cycle lengths are typically limited to 6-8 weeks. Liver support is recommended. Always consult with a healthcare professional before use.
1. Description — Clinical summary
Turinabol (chemical name chlorodehydromethyltestosterone, often abbreviated CDMT) is an oral, 17α‑alkylated anabolic–androgenic steroid (AAS). It was developed in the 1960s as a derivative of methandrostenolone (Dianabol) with a 4‑chloro substitution intended to reduce aromatization and androgenic effects while retaining anabolic properties. Turinabol has no widely accepted contemporary therapeutic indications in most countries and is best known from historical clinical use and its use for performance enhancement (including illicit use in sport). It is a controlled substance in many jurisdictions and is prohibited by most sports authorities.
Key points:
- Oral 17α‑alkylated AAS with primarily anabolic effects.
- Historically used experimentally; not an approved standard medicine in most countries.
- Associated with significant endocrine, hepatic and cardiovascular risks.
2. How does turinabol work? — Mechanism of action
- Turinabol is an androgen receptor (AR) agonist. It binds intracellular ARs in muscle and other tissues, promoting transcription of genes that increase protein synthesis, nitrogen retention, and muscle growth.
- The 4‑chloro modification reduces its conversion (aromatization) to estrogenic metabolites compared with some other AAS; therefore estrogen‑mediated effects (e.g., gynecomastia, fluid retention) are generally less prominent than with aromatizable steroids. However, estrogenic activity can still occur indirectly via complex endocrine changes.
- The 17α‑alkyl group permits effective oral administration by reducing first‑pass hepatic metabolism. This modification also confers an increased risk of hepatotoxicity (cholestasis, elevated liver enzymes, and, in rare cases, hepatic tumors).
- Exogenous androgen administration suppresses the hypothalamic–pituitary–gonadal (HPG) axis via negative feedback, causing reduced endogenous testosterone production, testicular atrophy, and reduced spermatogenesis while the drug is used.
3. Dosage — Medical and varying usage guidelines
Important legal/clinical note: Turinabol is not an approved medication in many jurisdictions and has no generally accepted medical dosing guidelines. The information below describes patterns reported in the scientific and forensic literature and in non‑medical contexts; it is not a recommendation or prescription. Use of AAS outside a licensed medical indication may be illegal and harmful. If there is any medical need to evaluate androgen therapy, consult a licensed physician or endocrinologist.
- Medical/approved use: No widely accepted contemporary therapeutic dosing regimen. Where used historically or experimentally, formal, evidence‑based dosing guidance is lacking.
- Reported non‑medical / performance‑enhancement use (informational/harm‑reduction context):
- Low/moderate reported daily oral dosages: approximately 5–40 mg/day.
- Typical “commonly reported” ranges in athlete reports: 20–50 mg/day.
- Some users report higher doses (above 50 mg/day) or combination with other AAS; higher doses increase risk of adverse effects substantially.
- Cycle lengths reported: commonly 6–8 weeks, sometimes up to 12 weeks; extended use increases hepatotoxicity and endocrine suppression risk.
- Women: much lower dosages reported (e.g., 2.5–10 mg/day) due to high risk of virilization; however even low doses can cause irreversible virilizing effects in some women.
- Special considerations:
- Because exogenous turinabol suppresses endogenous testosterone, monitoring and clinical follow‑up are important if any androgen therapy is under consideration.
- Concurrent use with other AAS, pharmaceuticals, or supplements increases complexity and risk (drug interactions, additive toxicities).
- Any intent to use AAS should be discussed with a healthcare professional; do not self‑prescribe.
Monitoring (recommended if exposure occurs or is medically supervised):
- Baseline and periodic liver function tests (ALT, AST, bilirubin, alkaline phosphatase).
- Lipid profile (HDL, LDL, triglycerides).
- Blood pressure.
- Serum total testosterone, LH, FSH; in men, semen analysis if fertility is a concern.
- Hematocrit/hemoglobin.
- Age‑appropriate prostate monitoring (PSA) in older men.
4. Side effects — Common and rare adverse effects
Adverse effects can be dose‑related and are more likely with higher doses and prolonged use.
Common/semi‑common adverse effects:
- Hepatic:
- Elevated liver enzymes (AST/ALT).
- Cholestatic liver injury, jaundice in some cases.
- Endocrine/reproductive:
- Suppression of HPG axis → decreased endogenous testosterone, reduced libido, testicular atrophy, decreased sperm production and potential infertility.
- Menstrual irregularities in women; virilization (voice deepening, hirsutism, clitoromegaly) can occur and may be irreversible.
- Cardiovascular/metabolic:
- Dyslipidemia — significant decrease in HDL cholesterol and increase in LDL cholesterol; increased cardiovascular risk with long‑term use.
- Hypertension.
- Androgenic:
- Acne, oily skin.
- Male pattern baldness (in genetically predisposed individuals).
- Neuropsychiatric:
- Mood changes, irritability, aggression, and mood swings.
- Others:
- Fluid retention (less than with aromatizing AAS but possible).
- Headaches.
Less common / rare but serious adverse effects:
- Severe hepatotoxicity including hepatic failure or rare hepatic tumors (hepatocellular adenoma/carcinoma) and peliosis hepatis — 17α‑alkylated AAS have been associated with these rare events.
- Thromboembolic events (reported with some AAS).
- Significant cardiovascular events (myocardial infarction, stroke) — especially with long‑term or high‑dose use and in presence of dyslipidemia or hypertension.
- Severe, persistent virilization in women.
- Prolonged or incomplete recovery of normal HPG axis function after cessation in some users.
Contraindications (typical):
- Pregnancy and breastfeeding (virilization of female fetus/infant).
- Known or suspected prostate or breast cancer.
- Pre‑existing severe hepatic disease.
- Uncontrolled cardiovascular disease.
- Children and adolescents (can impair growth).
Drug interactions and cautions:
- Co‑use with other hepatotoxic medications increases risk of liver injury.
- May alter metabolism of concomitant drugs (CYP-mediated interactions).
- May affect anticoagulant (warfarin) activity — monitor coagulation if necessary.
5. Storage — How to store it
If handling a pharmaceutical formulation (tablet form):
- Store at controlled room temperature, typically 15–30 °C (59–86 °F) unless the product labeling specifies otherwise.
- Protect from excessive heat, moisture and light.
- Keep in the original container with the lid tightly closed.
- Keep out of reach of children and pets.
- Do not use after the expiration date printed on the product.
- Proper disposal: follow local regulations for medication disposal; do not flush down the toilet. Many communities have medication take‑back programs or authorized disposal sites.
Final safety note
- Turinabol is a potent androgenic/anabolic agent with significant potential for harm. It is a controlled substance in many places and banned in sport. Any exposure or therapeutic consideration should involve a qualified healthcare professional; if unintentional or recreational use has occurred and there are concerning symptoms (jaundice, severe abdominal pain, chest pain, shortness of breath, marked mood changes, very high blood pressure), seek immediate medical attention.
1. Description — Clinical summary
Turinabol (active compound: chlorodehydromethyltestosterone, often abbreviated CDMT) is a synthetic anabolic–androgenic steroid (AAS). Chemically it is a 4-chloro derivative of methandienone with a 17α‑methyl group that allows oral activity. It was developed and used in some countries in the mid‑20th century and became well known for non‑medical performance uses. Turinabol is not widely approved for modern therapeutic indications in many jurisdictions and is classified as a controlled substance in many countries.
Pharmacologically it is an androgen receptor agonist with a relatively higher anabolic to androgenic activity than testosterone, and with low aromatization to estrogens. Because of its 17α‑alkylation, it is orally active but has potential for hepatotoxicity.
Clinical/therapeutic uses historically reported (limited modern medical use)
- Occasionally considered in contexts of catabolic states, severe muscle wasting or chronic illness in older literature.
- There are no widely accepted contemporary therapeutic guidelines or approved indications in many regulatory jurisdictions; modern medical use is rare.
Legal/regulatory note: Turinabol is regulated in many countries. Use outside a prescription or approved clinical setting is illegal and associated with health risks.
2. How does turinabol work? — Mechanism of action
- Androgen receptor activation: Turinabol binds to the androgen receptor (AR) in target tissues. The ligand–receptor complex translocates to the nucleus and alters transcription of androgen‑responsive genes, increasing protein synthesis and muscle anabolism.
- Anabolic vs androgenic profile: Chemical modifications (4‑chloro substitution) reduce the compound’s tendency to convert to estrogen (aromatize). This yields a relatively higher anabolic effect with lower estrogenic side effects (e.g., less gynecomastia) compared with aromatizable AAS.
- Protein and nitrogen balance: By stimulating AR signaling in muscle, turinabol increases nitrogen retention and net protein synthesis and reduces catabolism, which can lead to gains in lean mass and strength.
- Hypothalamic–pituitary–gonadal (HPG) axis suppression: Like other exogenous androgens, turinabol suppresses endogenous luteinizing hormone (LH) and follicle‑stimulating hormone (FSH) via negative feedback, reducing testicular testosterone production and sperm production.
- Hepatic metabolism: The 17α‑alkylation prolongs oral bioavailability but also predisposes to direct hepatotoxic effects; the liver is a primary site of metabolic transformation and potential organ toxicity.
Pharmacokinetics (reported/typical)
- Route: oral.
- Absorption: orally bioavailable due to 17α‑alkylation.
- Half‑life: reported estimates vary; many sources report an elimination half‑life on the order of hours to a day (estimates around ~16 hours have been reported), but specific values depend on assay and metabolites.
- Metabolism: hepatic, with formation of long‑lived metabolites detectable by specialized assays.
- Excretion: primarily renal after hepatic metabolism.
3. Dosage — Medical and varying usage guidelines
Important medical caveat: Turinabol has limited to no current mainstream, approved therapeutic indications in many countries. The doses below summarize reported historical/empirical ranges and non‑medical (recreational/performance) use patterns described in the literature and on harm‑reduction resources. This is informational only — any use should be under a licensed clinician’s supervision where legal and medically appropriate.
Medical/therapeutic dosing (historical, limited data)
- Reported therapeutic doses in older literature or compassionate use: low single‑digit to low‑teens of milligrams daily (for example, 2.5–10 mg/day).
- Duration: when used therapeutically, durations were typically short and monitored closely because of hepatic and endocrine risks.
Commonly reported performance/illicit use patterns (NOT a recommendation)
- Men (typical reports): 20–50 mg orally per day. Some users report shorter cycles (4–8 weeks) to limit hepatotoxic exposure.
- Women (typical reports): 2.5–10 mg per day (very conservative), due to risk of virilization. Many clinicians advise against use because of irreversible masculinizing effects.
- Cycle length: commonly limited to 4–8 weeks in practice for harm‑reduction reasons; longer use increases liver and endocrine risks.
- Stacking: often combined with other AAS (testosterone esters, nandrolone, etc.) in illicit settings; this increases complexity and risk (additive suppression, hepatic strain, cardiovascular risk).
Monitoring and safety measures (recommended if ever used clinically)
- Baseline assessment: liver function tests (AST, ALT, bilirubin), fasting lipid profile, complete blood count (CBC), blood pressure, serum testosterone (men), renal function, and — for older men — PSA.
- Serial monitoring: LFTs and lipids every 2–4 weeks while on a course; blood pressure and symptoms at regular intervals. If transaminases rise significantly (e.g., >2–3× upper limit of normal) or cholestatic signs occur, discontinue and evaluate.
- Post‑course: monitor endogenous testosterone recovery; evaluate need for endocrine follow‑up if symptoms of hypogonadism persist.
Contraindications (major)
- Known or suspected prostate or breast cancer (in men).
- Pregnancy or breastfeeding (risk of fetal virilization).
- Active or severe hepatic disease or significant baseline liver enzyme elevation.
- Uncontrolled cardiovascular disease.
- Adolescents (risk of premature epiphyseal closure and endocrine disruption).
Drug interactions and cautions
- Concurrent hepatotoxic drugs increase liver injury risk (e.g., high doses acetaminophen, some antifungals, certain antivirals, macrolide antibiotics — check specifics).
- May alter lipid profiles (lower HDL, raise LDL) and interact with lipid‑modifying agents or anticoagulants; monitor closely.
- Concomitant use with other androgens or corticosteroids increases adverse event risk.
Special populations
- Women: high risk of virilizing effects (deepening voice, clitoromegaly) — use generally discouraged; if used, very low doses and rapid cessation at first signs of virilization.
- Elderly: increased cardiovascular risk; careful assessment required.
Because of the legal status and safety concerns, any administration should be done only with appropriate clinical indication, informed consent, and monitoring.
4. Side effects — Common and rare adverse effects
Common/expected adverse effects
- Hepatic toxicity: as a 17α‑alkylated oral steroid, turinabol can cause elevated transaminases, cholestasis, and, rarely, clinically significant liver injury.
- Endocrine suppression: decreased LH/FSH and suppressed endogenous testosterone production; symptoms may include low libido, erectile dysfunction, fatigue, and testicular atrophy.
- Dyslipidemia: decreased HDL cholesterol and increased LDL cholesterol, contributing to atherogenic risk.
- Acne and oily skin.
- Hair effects: accelerated male‑pattern hair loss in genetically predisposed men.
- Fluid retention and potential increases in blood pressure (less pronounced than with strongly aromatizing AAS but possible).
- Virilization in women (voice deepening, hirsutism, clitoral enlargement, menstrual irregularities).
Less common but serious/rare adverse effects
- Cholestatic jaundice and clinically significant liver injury; in rare cases, hepatic tumors (adenomas or carcinomas) and peliosis hepatis have been associated with long‑term 17α‑alkylated AAS use.
- Venous thromboembolism (case reports with AAS usage).
- Cardiovascular events: myocardial infarction, stroke — likely mediated through dyslipidemia, hypertension, and prothrombotic changes.
- Psychiatric effects: mood changes, aggression, irritability, depressive symptoms on withdrawal.
- Fertility impairment: prolonged suppression of spermatogenesis; recovery may be delayed and sometimes incomplete after prolonged use.
- Gynecomastia is less common due to minimal aromatization but can occur indirectly through hormonal imbalances or when combined with other aromatizing agents.
Signs requiring immediate medical attention
- Jaundice, dark urine, severe abdominal pain (possible hepatic injury).
- Chest pain, shortness of breath, sudden weakness or slurred speech (cardiovascular or cerebrovascular event).
- Sudden severe mood changes or suicidal ideation.
- Rapid or unexplained swelling, severe hypertension.
Reporting and monitoring
- Any significant elevations in liver enzymes, persistent endocrine symptoms, or cardiovascular symptoms should prompt immediate discontinuation and medical evaluation.
5. Storage — How to store it
(These instructions apply to oral tablet formulations or sealed pharmaceutical packaging. Follow manufacturer labeling when available.)
- Temperature: store at controlled room temperature, generally 20–25 °C (68–77 °F). Short excursions may be acceptable per product labeling (e.g., 15–30 °C), but avoid extremes of heat and cold.
- Light and moisture: keep in the original tightly closed container to protect from light and moisture. Do not store in bathrooms or other humid locations.
- Child safety: keep out of reach and sight of children and pets.
- Expiration: do not use beyond the manufacturer’s expiration date. Dispose of expired or unwanted medication following local regulations or pharmacy take‑back programs.
- Transport: keep in original packaging when transporting; avoid leaving in a hot car or in direct sunlight.
- Disposal: follow local guidelines for pharmaceutical disposal; do not flush down the toilet or discard in household trash if not permitted.
Final notes and clinical caution
- Turinabol is associated with significant medical risks, particularly hepatotoxicity, endocrine disruption, and adverse cardiovascular/lipid effects. It has little contemporary medical use and is widely used illicitly for performance enhancement.
- If a clinician considers its use where legally permitted, only use with a clear therapeutic indication, informed consent, appropriate baseline evaluation and ongoing monitoring.
- If you are taking any androgenic agents or considering therapy, discuss risks, alternatives, and monitoring with a licensed healthcare professional.