UNIQUE PHARMA QUALITY: Premium pharmaceutical grade Testosterone Propionate (Test Prop) manufactured under strict GMP conditions with 99.8% purity verification.
Test Prop from Unique Pharma represents our commitment to delivering exceptional quality performance enhancement compounds. Each batch undergoes rigorous testing to ensure consistent potency and purity standards.
Key Characteristics of Test Prop
This injectable compound is administered via intramuscular injection and remains active in your system for approximately 4.5 Days. Notable features include:
- Pharmaceutical grade manufacturing
- Batch-tested for purity and potency
- Consistent dosing per unit
- Optimal bioavailability
Primary Benefits:
- Enhanced performance and recovery
- Quality-assured formulation
- Reliable and consistent results
- Professional-grade compound
Mechanism of Action
Testosterone Propionate works by interacting with androgen receptors in muscle tissue, promoting protein synthesis and nitrogen retention. This creates an optimal environment for muscle development and recovery. The compound's unique molecular structure provides specific benefits that distinguish it from other options in its class.
Usage Guidelines
Unique Pharma Test Prop is suitable for experienced users who understand proper cycling protocols. Always consult with a healthcare professional before beginning any supplementation regimen. Proper post-cycle therapy should be considered based on individual needs and cycle duration.
Recommended Applications
This compound is commonly incorporated into both bulking and cutting protocols depending on the user's specific goals. Its versatility makes it a popular choice among athletes and bodybuilders seeking reliable results.
Potential Considerations
As with any performance compound, users should be aware of potential effects and monitor their response accordingly. Regular health monitoring is recommended during use. Individual responses may vary based on genetics, diet, training, and other factors.
Quality Assurance
Every Unique Pharma product undergoes comprehensive quality control including:
- Raw material verification
- In-process testing
- Final product analysis
- Stability testing
Warning: Keep out of reach of children. For adults only. Not intended for use by individuals under 18 years of age.
Related products
Other Unique Pharma products
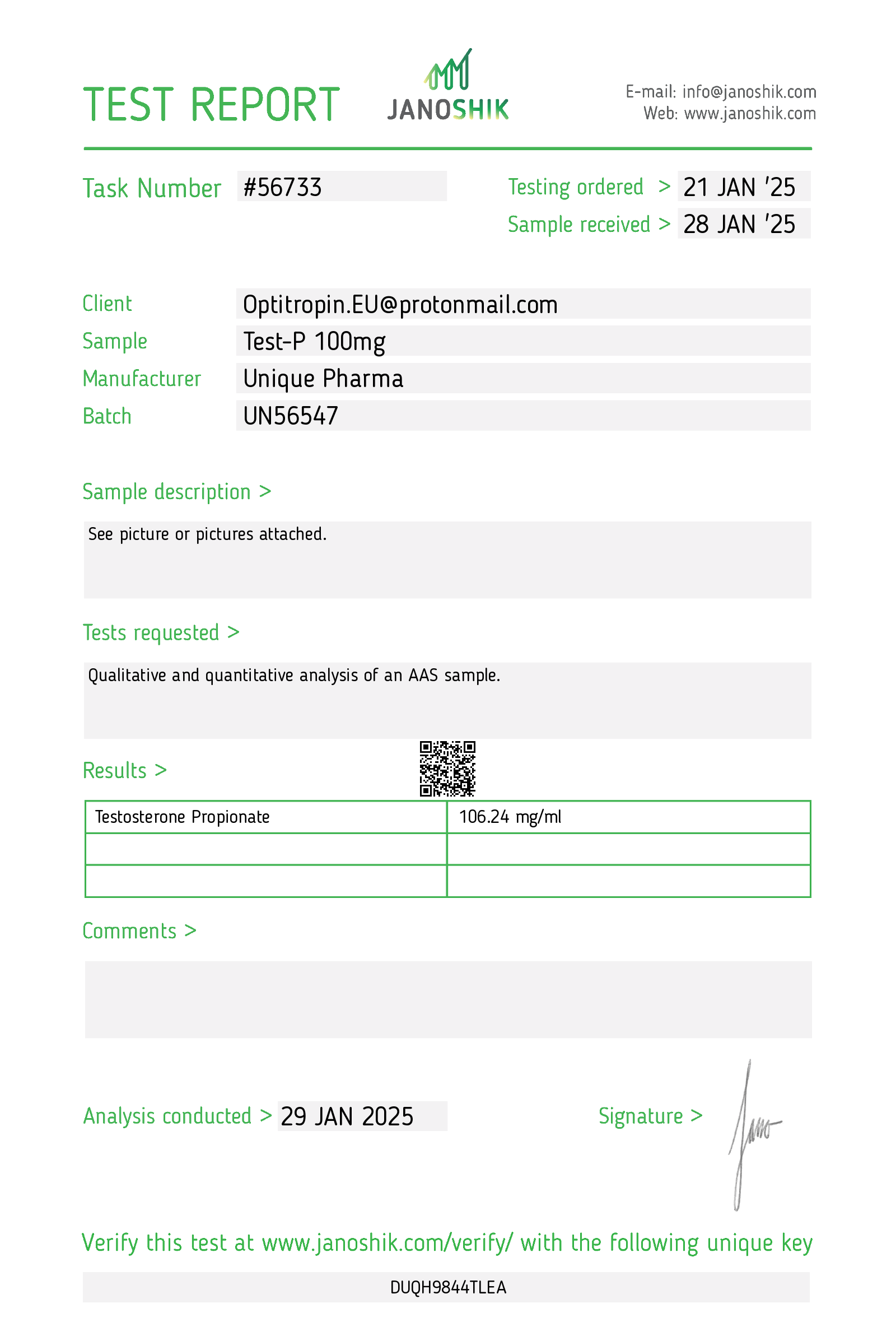
1. Description
Test P 100 mg/mL (active compound: testosterone propionate) is an intramuscular (IM) injectable androgen formulation in which testosterone is chemically linked to the short-chain propionate ester. It is indicated for replacement therapy in adult males with primary or secondary hypogonadism when testosterone deficiency has been confirmed by clinical features and laboratory testing. Testosterone propionate produces physiologic androgenic effects (development/maintenance of male secondary sexual characteristics, anabolic effects on protein metabolism) and is also used in selected clinical situations under specialist supervision (e.g., certain delayed puberty cases, cachexia related to catabolic states) where short-acting injectable testosterone is preferred.
Testosterone propionate is a prescription medication and a controlled substance in many jurisdictions. Use should be supervised by a qualified clinician with appropriate baseline evaluation and periodic monitoring.
2. How does testosterone-propionate work?
- Testosterone propionate is an esterified form of testosterone designed for intramuscular depot administration. After IM injection, local esterases cleave the propionate ester and release free testosterone into the circulation.
- Free testosterone binds to androgen receptors in target tissues (muscle, bone, prostate, hair follicles, skin, brain), modulating gene transcription and leading to androgenic and anabolic effects: development/maintenance of male secondary sexual characteristics, stimulation of erythropoiesis, increased nitrogen retention and protein synthesis.
- Testosterone is also aromatized to estradiol in peripheral tissues by aromatase; estradiol mediates some effects (e.g., bone health, feedback on the hypothalamic–pituitary axis).
- Exogenous testosterone suppresses the hypothalamic–pituitary–gonadal axis (decreased GnRH → decreased LH/FSH), which can reduce intratesticular testosterone production and impair spermatogenesis (possible reversible infertility).
- Pharmacokinetics: as a short-acting ester, testosterone propionate reaches higher peak levels sooner and clears faster than longer esters (enanthate, cypionate). This typically requires more frequent injections (every 48–72 hours) to maintain more stable serum concentrations.
3. Dosage
All dosing should be individualized and prescribed by a clinician. The dose and frequency depend on the indication, patient characteristics, and monitoring results.
General medical dosing (adults):
- Hypogonadism (male replacement therapy): commonly 50–100 mg IM every 48–72 hours (many clinicians use 50 mg every other day or 100 mg every 2–3 days). The aim is to restore serum testosterone to the mid-normal physiologic range (measured in the morning) and relieve symptoms.
- Alternative regimens: some clinicians use lower divided daily dosing (e.g., 25–50 mg daily) to reduce peak–trough variation, but more frequent injections may be required due to the short ester.
- Transgender men: testosterone propionate has been used in similar dosing ranges (e.g., 50–100 mg every 2–3 days). Choice of preparation and regimen should be individualized and supervised by an experienced clinician or gender health program.
- Women: testosterone therapy in women is limited, used cautiously and at much lower doses for specific indications (e.g., hypoactive sexual desire disorder) and usually with formulations and doses different from those used in men. Injectable testosterone propionate is generally not recommended for routine female indications because of virilization risk.
- Pediatrics: use in children is reserved for specific indications (e.g., delayed puberty) and must be under pediatric endocrinology guidance with lower, carefully titrated doses.
Important clinical points and adjustments:
- Monitor serum testosterone (morning), hematocrit/hemoglobin, PSA (in men), liver function, lipids, and blood pressure. Adjust dose based on clinical response and laboratory findings.
- For hypogonadal men, check serum testosterone 2–3 days after an injection (for propionate) to assess trough and peak. Goal is physiologic replacement without supraphysiologic peaks.
- If hematocrit rises above 54% (or per local lab/clinic thresholds), reduce dose, extend dosing interval, or interrupt therapy; consider evaluation for secondary causes and possible therapeutic phlebotomy.
- Use with caution and specialist input in patients with cardiovascular disease, sleep apnea, uncontrolled heart failure, or elevated thrombotic risk.
Non-medical/supraphysiologic use:
- Supraphysiologic doses used for performance enhancement are associated with markedly increased risk of adverse events, suppression of fertility, and are illegal in many jurisdictions. This guide does not recommend or provide regimens for non-medical use. Patients should be counseled about risks and encouraged to seek medical care.
Administration technique (brief):
- Administer only by deep intramuscular injection (e.g., gluteal or deltoid muscle) using aseptic technique. Do not inject intravenously.
- Typical needle sizes: gluteal IM—21–23 gauge, 1–1.5 inch; deltoid IM—22–25 gauge, 1 inch; adjust for body habitus. Rotate injection sites.
- If using multi-dose vials, follow manufacturer instructions and sterile technique. Observe for particulate matter or discoloration; do not use if present.
4. Side effects
Common and predictable effects:
- Injection-site pain and local reactions (propionate ester is often associated with more local discomfort than longer esters).
- Androgenic effects: acne, oily skin, increased facial/body hair, male-pattern hair growth, scalp hair loss in those predisposed.
- Fluid retention and weight gain.
- Increased hematocrit/hemoglobin and risk of polycythemia.
- Changes in lipid profile: decreased HDL cholesterol and possible adverse effects on LDL/triglycerides.
- Mood and behavioral changes: irritability, aggression, mood lability; some patients report increased libido.
- Suppression of spermatogenesis and testicular atrophy with long-term use.
Less common but serious adverse effects:
- Prostate effects: stimulation of benign prostatic hyperplasia (BPH) symptoms and potential exacerbation of undiagnosed prostate cancer. Testosterone therapy is contraindicated in men with known or suspected prostate cancer.
- Cardiovascular: increased risk of hypertension, possible association with adverse cardiovascular events in some populations (data mixed); use caution in patients with established cardiovascular disease.
- Thromboembolic events: risk of venous thromboembolism has been reported.
- Hepatic effects: injectable testosterone has lower hepatotoxicity risk than some oral 17-alkylated androgens, but liver function should be monitored.
- Allergic reactions: rare hypersensitivity to active ingredient or excipients.
- Virilization in women and female fetuses: deepening of voice, clitoromegaly, menstrual irregularities; severe risk of fetal virilization if used during pregnancy.
Drug interactions:
- May alter effects of anticoagulants (monitor INR if on warfarin).
- May affect blood glucose and insulin sensitivity—monitor diabetic patients when initiating or changing dose.
- Concurrent use with other androgens/anabolic agents increases risk of adverse effects.
Contraindications:
- Known or suspected prostate or breast cancer.
- Pregnancy and breastfeeding (can cause fetal virilization).
- Hypersensitivity to testosterone or formulation excipients.
- Uncontrolled severe heart failure or prior thrombosis, depending on clinician judgment.
Patient counseling:
- Discuss fertility implications (possible reversible infertility), contraception if appropriate, need for regular monitoring, signs and symptoms that require immediate care (e.g., chest pain, shortness of breath, sudden visual changes, signs of thrombotic events).
5. Storage
- Store at controlled room temperature as specified by the manufacturer. Typical recommended storage: 20–25 °C (68–77 °F) with permitted excursions to 15–30 °C (59–86 °F). Follow the product label for exact storage conditions.
- Protect from light and heat. Keep the vial in its original carton until use.
- Do not freeze. If the product contains suspensions or oil-based solutions, cold temperatures can alter viscosity or depot characteristics.
- Inspect the solution before use: do not use if there is cloudiness (unless specified by manufacturer), particulate matter, discoloration, or if the vial is damaged.
- Keep out of reach of children and pets.
- Dispose of used needles and syringes in an approved sharps container according to local regulation; do not recap used needles. Follow local rules for disposal of unused medication and containers.
Additional notes
- Prescribers should follow current clinical guidelines (endocrinology/urology/primary care) and local regulatory controls for initiation and monitoring of testosterone therapy.
- Any change in symptoms, new-onset breast changes, urinary symptoms, cardiovascular events, or concerns about fertility should prompt timely medical review.
1. Description — Clinical summary
Testosterone propionate (Test P) is a short‑acting injectable ester of testosterone. It delivers exogenous testosterone for replacement therapy in male hypogonadism and, less commonly, for selected off‑label indications under specialist supervision. As an androgenic–anabolic steroid, it produces both androgenic (masculinizing) and anabolic (protein‑sparing/protein‑building) effects. Because the propionate ester is short‑acting, injections are given frequently (usually every 48–72 hours) to maintain relatively stable serum testosterone.
Formulation referenced: “Test P 100 mg/mL” indicates 100 mg of testosterone propionate per mL of solution; usual administration is by intramuscular injection (deep IM). Use should be guided and supervised by a licensed clinician; monitoring and dose adjustment are required.
2. How does testosterone‑propionate work? — Mechanism of action
- Testosterone propionate is testosterone chemically linked to a propionate ester. After IM injection, ester hydrolysis releases free testosterone into the circulation.
- Free testosterone acts primarily by binding to the androgen receptor (AR) in target tissues (skeletal muscle, bone, brain, liver, prostate, skin, etc.), altering gene transcription and promoting androgenic and anabolic effects.
- In several tissues (skin, prostate, scalp), testosterone is converted by 5α‑reductase to dihydrotestosterone (DHT), a more potent AR agonist, increasing androgenic effects in those tissues.
- Testosterone increases protein synthesis, nitrogen retention, and erythropoiesis (stimulates red cell production via erythropoietin), and modulates lipid metabolism, bone density, libido, mood, and secondary sexual characteristics.
- Short ester = rapid rise and relatively short duration of action (typical dosing interval every 48–72 hours) compared with longer esters (cypionate, enanthate, undecanoate).
3. Dosage — Medical and varying usage guidelines
Important: Dosing must be individualized by a clinician based on indication, baseline testosterone levels, age, comorbidities, and response. The ranges below are typical reference values used clinically or reported in medical literature; they are not a substitute for medical prescription and monitoring.
General administration:
- Route: Intramuscular (deep gluteal or deltoid) injection. Rotate sites. Use proper sterile technique.
- Frequency: Because of the short ester, injections are commonly every 48–72 hours (every other day or every 2–3 days) to reduce fluctuations.
Typical dosing examples (approximate):
-
Adult males with hypogonadism (replacement therapy):
- Common: 50–100 mg IM every 48–72 hours (equivalent ~150–300 mg/week if dosed every other day).
- Some regimens use 25–50 mg every other day to mimic physiologic levels more closely; other clinicians give 100 mg every 2–3 days.
- Note: Longer‑acting esters (testosterone cypionate/enanthate/undecanoate) are more commonly used in many practices for convenience and more stable levels.
-
Women (very limited and specialist use):
- Injectable testosterone is rarely first‑line in women. When used (e.g., for severe hypoactive sexual desire disorder under specialist care), doses are much smaller and often via transdermal preparations are preferred.
- If injectable used, extremely low doses under specialist supervision; typical intramuscular doses reported are on the order of 2.5–10 mg at intervals, but injectables are rarely recommended for female therapy.
-
Pediatric use:
- Testosterone therapy in adolescents or children is complex and must be managed by pediatric endocrinologists. Dosing is individualized and initiated only for specific indications (delayed puberty, pubertal induction), with gradually increasing doses.
-
Off‑label/non‑medical use (bodybuilding, performance enhancement):
- Frequently reported doses are higher (e.g., 50–100 mg every other day or higher), often combined with other anabolic steroids. This use carries increased risk of adverse effects and is not medically recommended.
Dose adjustments and monitoring:
- Start at the lowest effective dose and titrate to clinical response and serum testosterone levels (aiming for mid‑physiologic range for the indication).
- Monitor trough levels (just before next injection) if level stability is a goal; adjust dose or frequency if peaks/troughs cause symptoms or lab abnormalities.
Special considerations:
- Fertility: Exogenous testosterone suppresses the hypothalamic‑pituitary‑gonadal axis and can markedly reduce spermatogenesis and fertility. Discuss reproductive plans before initiating therapy.
- Comorbidities: In men with cardiovascular disease, severe sleep apnea, uncontrolled heart failure, or polycythemia, therapy may be contraindicated or require caution and close monitoring.
4. Side effects — Common and rare adverse effects
Common / frequent:
- Injection‑site pain, swelling, erythema.
- Acne, oily skin, increased facial/body hair.
- Fluid retention and peripheral edema.
- Increased libido (or, less commonly, mood changes).
- Increased hematocrit/hemoglobin (polycythemia) — requires monitoring.
- Suppression of spermatogenesis and testicular atrophy with prolonged use.
- Worsening of benign prostatic hyperplasia (BPH) symptoms.
Less common / potentially serious:
- Gynecomastia (estrogenic effect secondary to aromatization of testosterone to estradiol; may require management).
- Psychiatric/behavioral changes: mood swings, aggression, irritability, depression in some users.
- Dyslipidemia: reductions in HDL cholesterol and potential adverse changes in LDL.
- Elevated blood pressure.
- Sleep apnea exacerbation.
Rare but serious:
- Thromboembolic events (deep vein thrombosis, pulmonary embolism) — reported with androgen therapy in susceptible individuals.
- Cardiovascular events: some observational studies suggest increased risk of myocardial infarction, stroke or other cardiovascular events in certain populations (eg, older men with preexisting cardiac disease); evidence is mixed. Use with caution and individualized risk assessment.
- Hepatic toxicity: oral 17α‑alkylated androgens carry higher liver risk; injectable testosterone esters have lower incidence of cholestatic hepatic injury but monitor LFTs as clinically indicated.
- Allergic reactions (rare): rash, anaphylaxis.
Contraindications and cautions:
- Known or suspected prostate cancer or breast cancer in males.
- Pregnant or breastfeeding women must not be exposed — virilization risk to female fetus/infant.
- Uncontrolled severe heart failure, hypertension, significant coronary artery disease — use with caution.
- Polycythemia (Hct >50%) is a relative contraindication until corrected.
When to seek urgent medical attention:
- Signs of deep vein thrombosis (leg swelling, pain), sudden shortness of breath, chest pain, or neurologic deficits.
- Severe allergic reaction (hives, difficulty breathing, facial swelling).
- Symptoms of markedly elevated hematocrit (headache, visual changes), or severe persistent mood changes.
Monitoring recommendations (typical clinical practice):
- Baseline and interval: serum total testosterone, hematocrit/hemoglobin, lipid profile, liver function tests, blood pressure.
- PSA and digital rectal exam baseline in men ≥40 (or earlier with risk factors) and periodically during therapy.
- Clinical assessment for efficacy and adverse effects at regular intervals (eg, every 3 months initially, then 6–12 months once stable), with more frequent checks for high‑risk patients.
5. Storage — How to store it
- Store at controlled room temperature as specified by the product labeling (commonly 20–25°C / 68–77°F). Brief excursions permitted within manufacturer guidance.
- Protect from light. Do not freeze.
- Keep in original container until use. Discard any product if particulate matter or discoloration is seen (follow product instructions).
- Keep out of reach of children and pets.
- For single‑use vials, do not reuse needles or syringes. Follow local regulations for safe disposal of sharps and pharmaceutical waste.
- If any specific storage instructions appear on the product leaflet (refrigeration, maximum shelf life after opening), follow the manufacturer’s directions.
Final important notes
- Testosterone propionate is a prescription medication. Use only under the supervision of a qualified clinician.
- Individual risk/benefit assessment and appropriate baseline and ongoing monitoring are essential.
- Discuss fertility plans and potential effects on spermatogenesis before starting therapy.
- This guide is educational and not a substitute for personalized medical advice. If you or a patient are considering or currently using testosterone propionate, consult an endocrinologist, urologist, or primary care physician experienced with androgen therapy for individualized care.